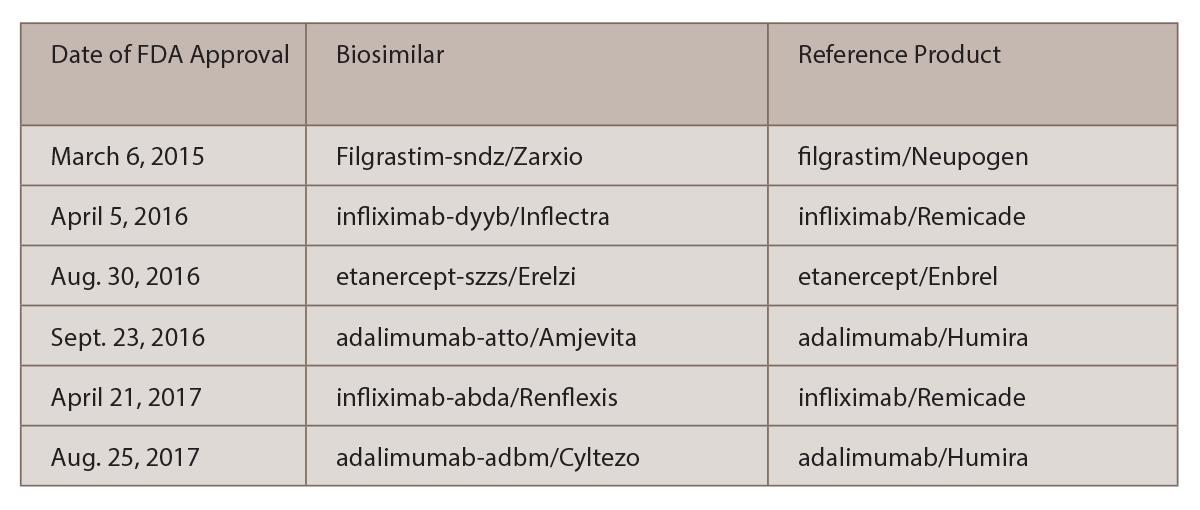
TNF-α inhibitor originators and FDA-approved biosimilars by the end of... | Download Scientific Diagram

Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn's disease on combination therapy (SPARE): a multicentre, open-label, randomised controlled trial - The Lancet Gastroenterology & Hepatology
Report on EU's Experience with Biosimilar Drugs Released: Will U.S. Experience Be Similar? - Page 2 of 5 - The Rheumatologist

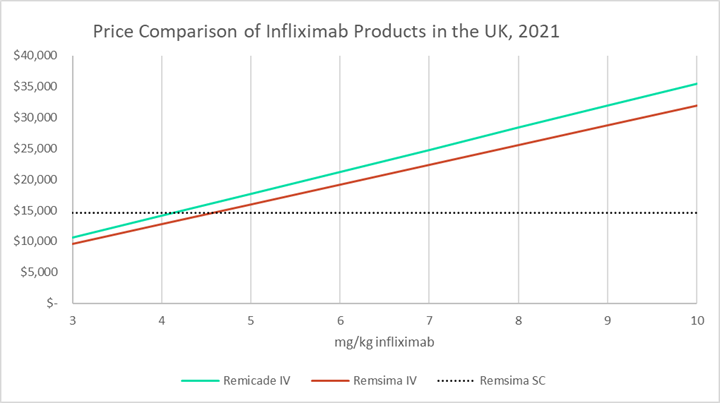
Biosimilar strategy for infliximab by MedDrive™ includes the reference drug - Prime Therapeutics LLC

Biosimilar medicine fact sheet – infliximab | Australian Government Department of Health and Aged Care